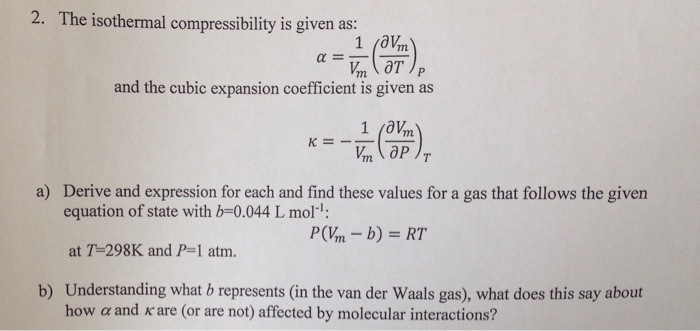Amazon.in: Buy Compressibility Considerations for Kappa-Omega Turbulence Models in Hypersonic Boundary Layer Applications Book Online at Low Prices in India | Compressibility Considerations for Kappa-Omega Turbulence Models in Hypersonic Boundary Layer ...
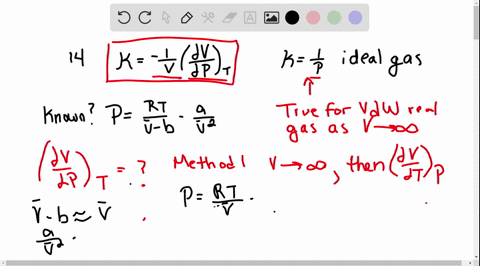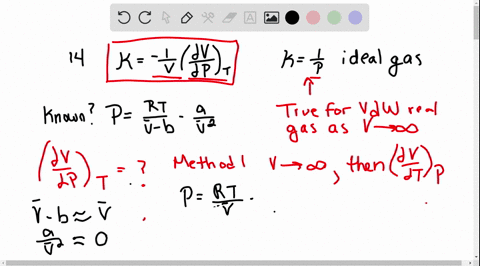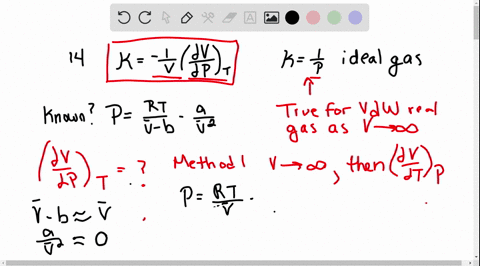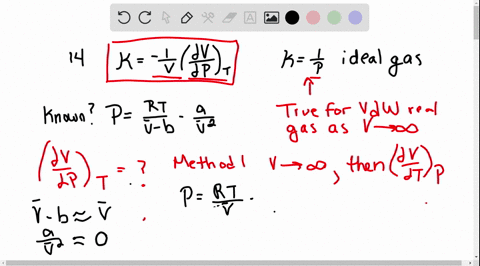
SOLVED:The isothermal compressibility κof a gas is defined in Problem 1.17, and its value for an ideal gas is shown to be 1 / P Use implicit differentiation of V with respect

The compressibility kappa of a substance is defined as the fractional change in volume of that substance for a given change in pressure : kappa = - 1V dVdP (a) Explain why

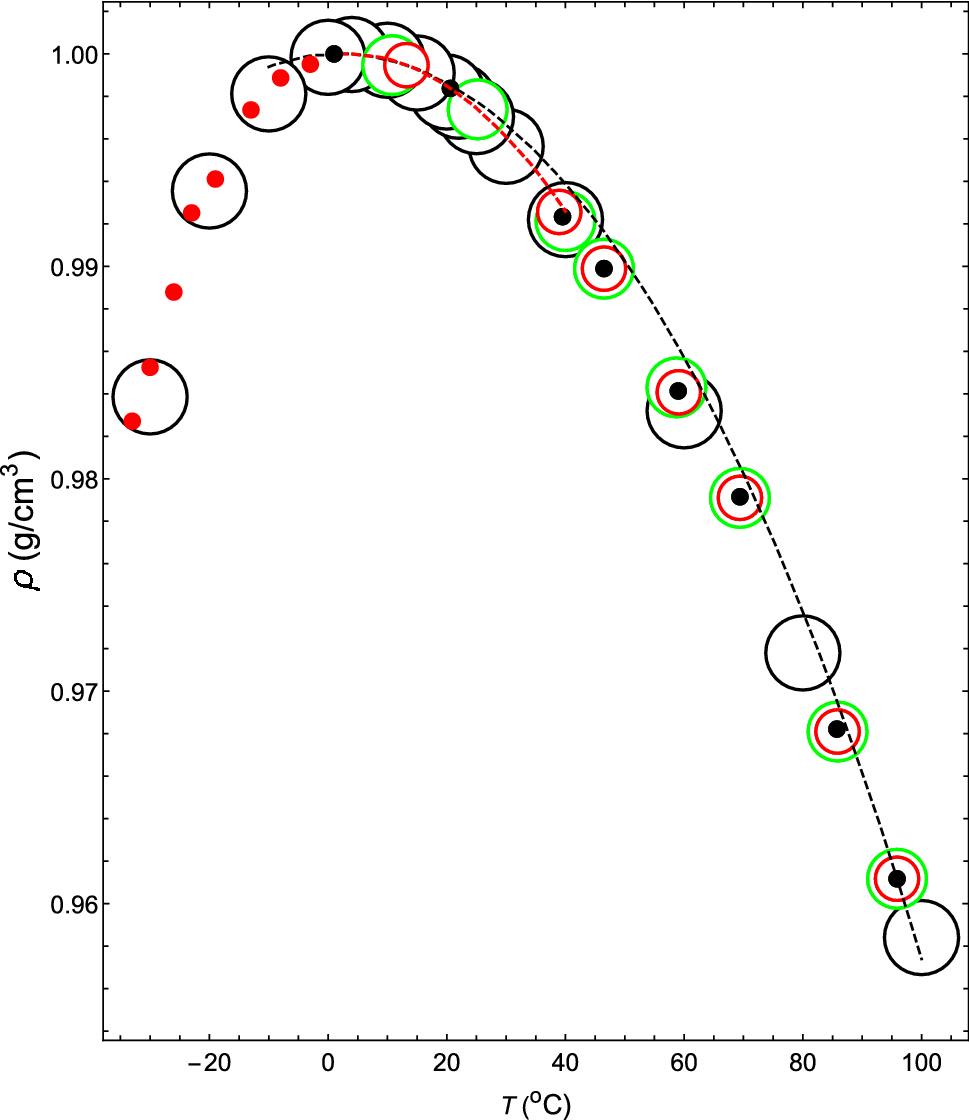
The average compressibility $\overline{\kappa }$ as a function of Δ... | Download Scientific Diagram
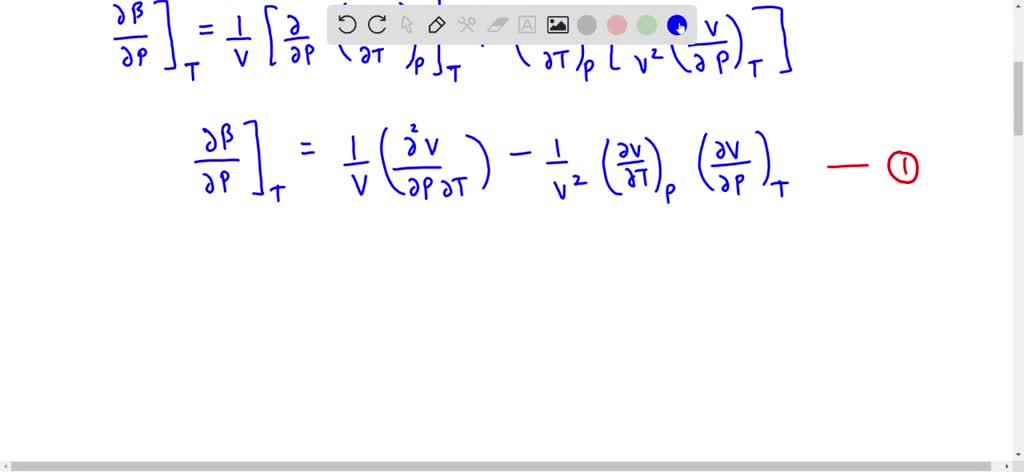
SOLVED:Generally, volume expansivity βand isothermal compressibility κdepend on T and P. Prove that: ((∂β)/(∂P))T=-((∂κ)/(∂T))P

The compressibility kappa of a substance is defined as the fractional change in volume of that substance for a given change in pressure : kappa = - 1V dVdP (a) Explain why

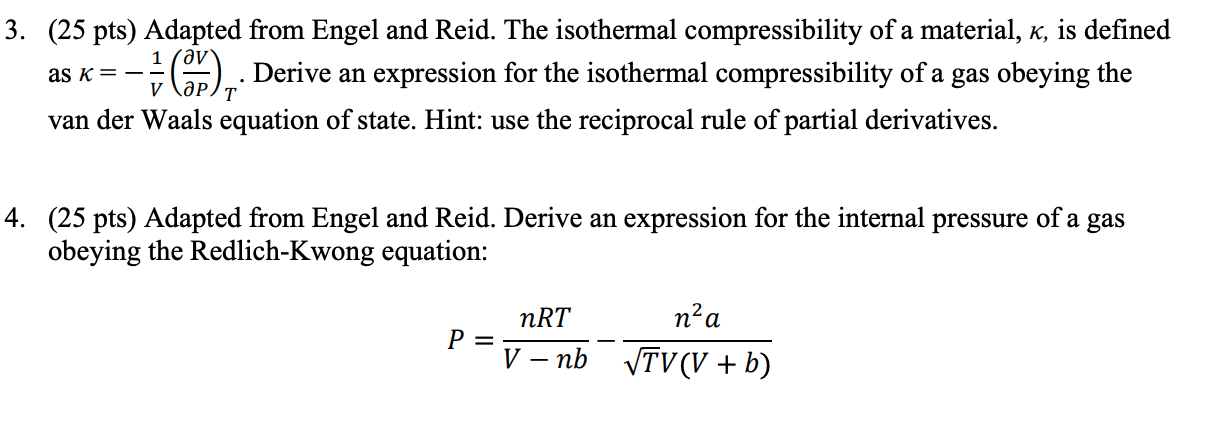
SOLVED: Isothermal compressibility, Kr (kappa defined as: Derive the formula that - van der waals gas would have for kappa Show the unit analysis on your answer in part and check if
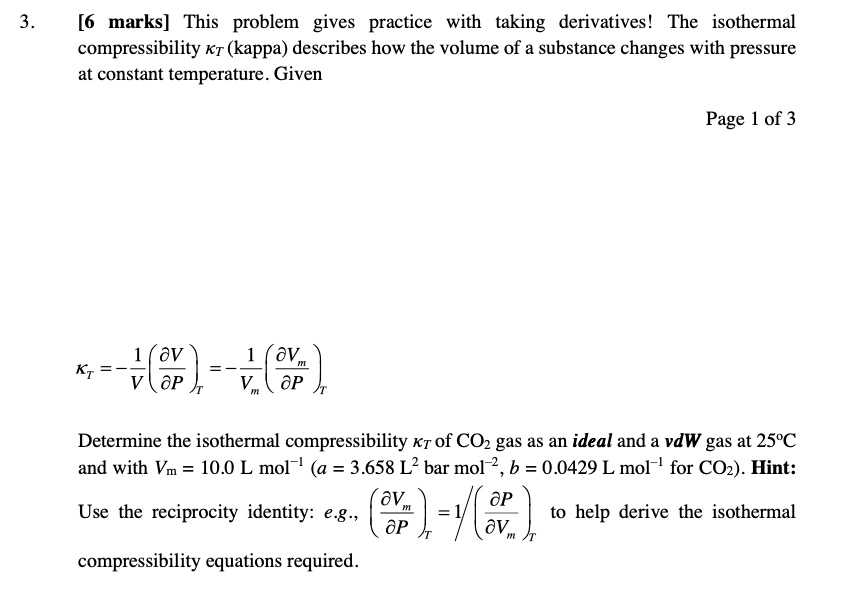
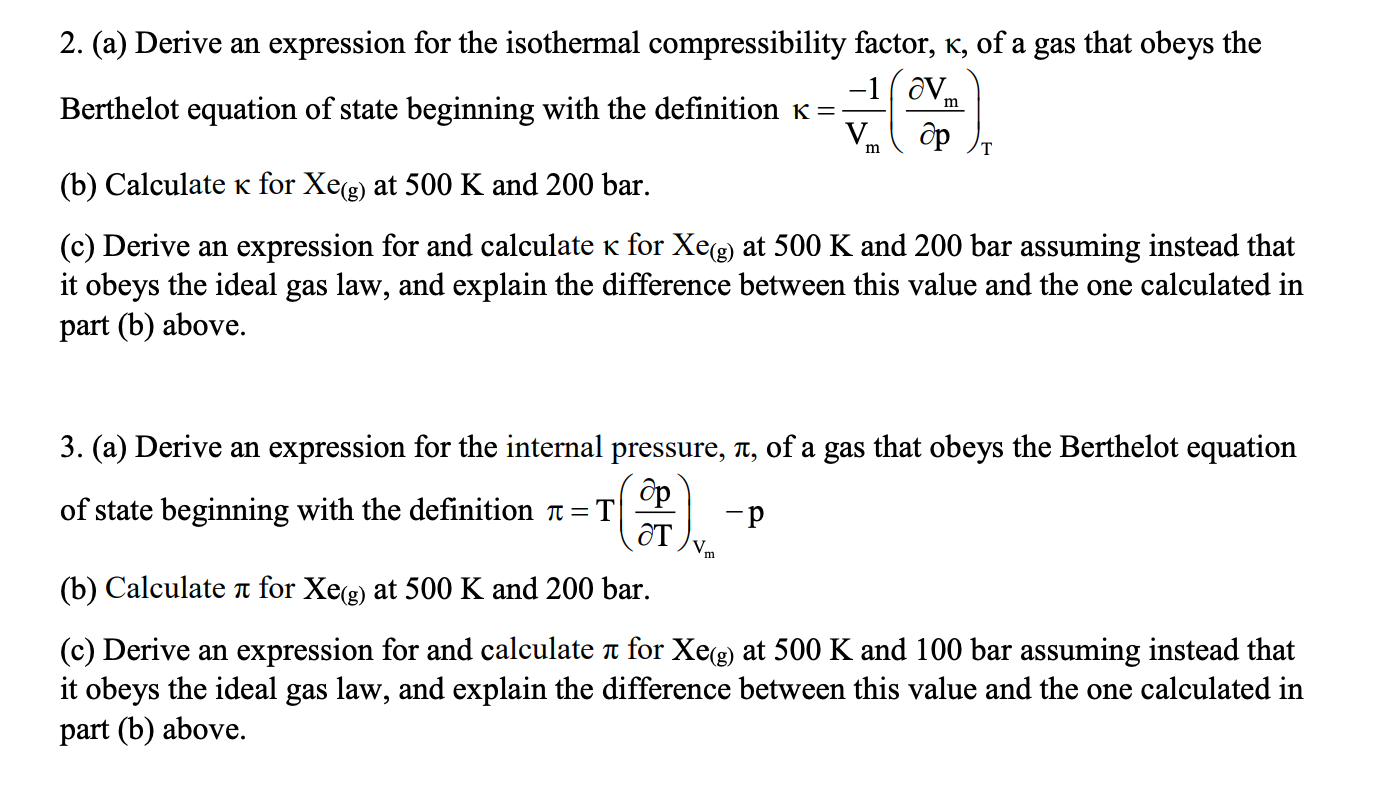
SOLVED: This problem gives practice with taking derivatives! The isothermal compressibility Kr (kappa) describes how the volume of a substance changes with pressure at constant temperature. Given KT = -X(av) = -V (
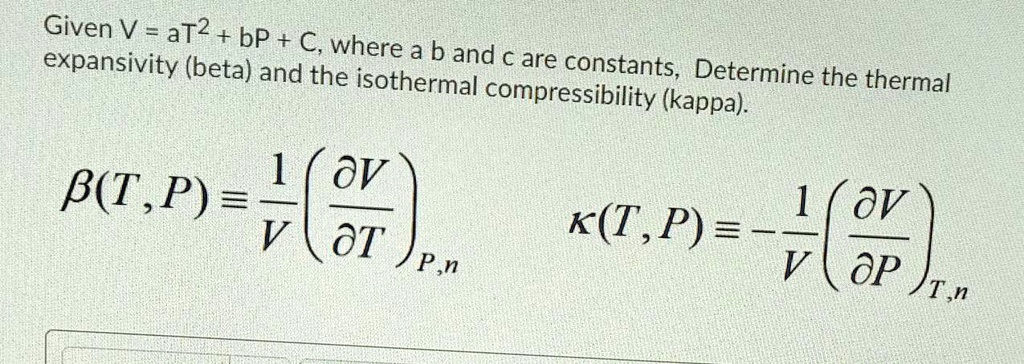
SOLVED: Given V = aT2 + bP + C, where expansivity (beta) a b and € are constants, and the isothermal Determine the thermal compressibility (kappa): oV B(T ,P) = ov V
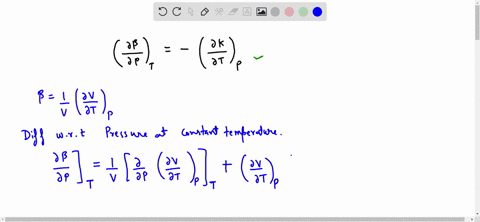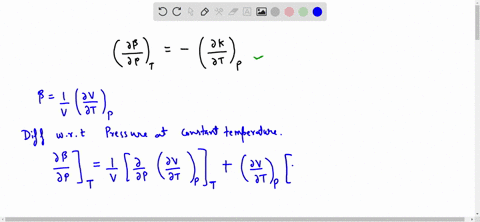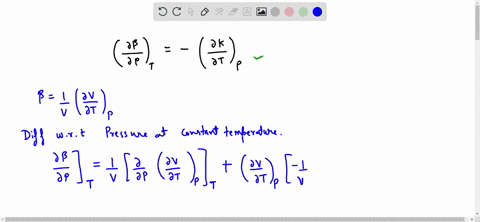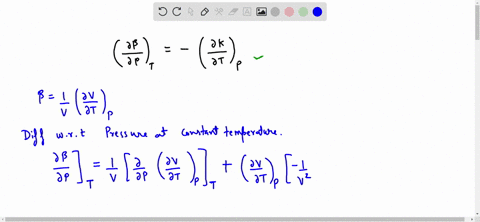
SOLVED:Generally, volume expansivity βand isothermal compressibility κdepend on T and P. Prove that: ((∂β)/(∂P))T=-((∂κ)/(∂T))P
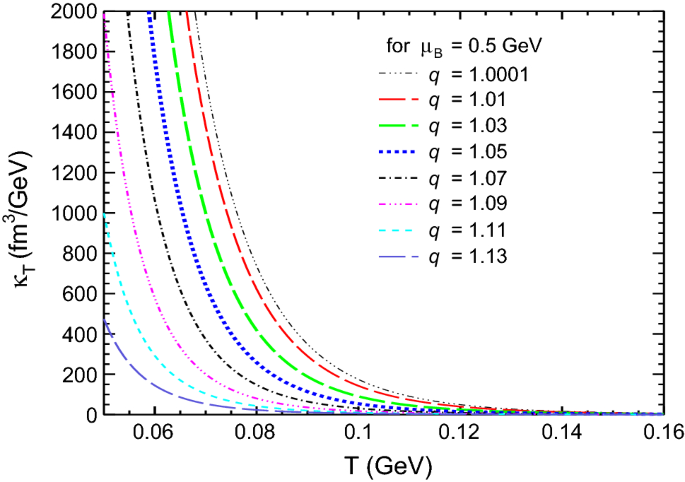
Dissipative properties and isothermal compressibility of hot and dense hadron gas using non-extensive statistics | The European Physical Journal C